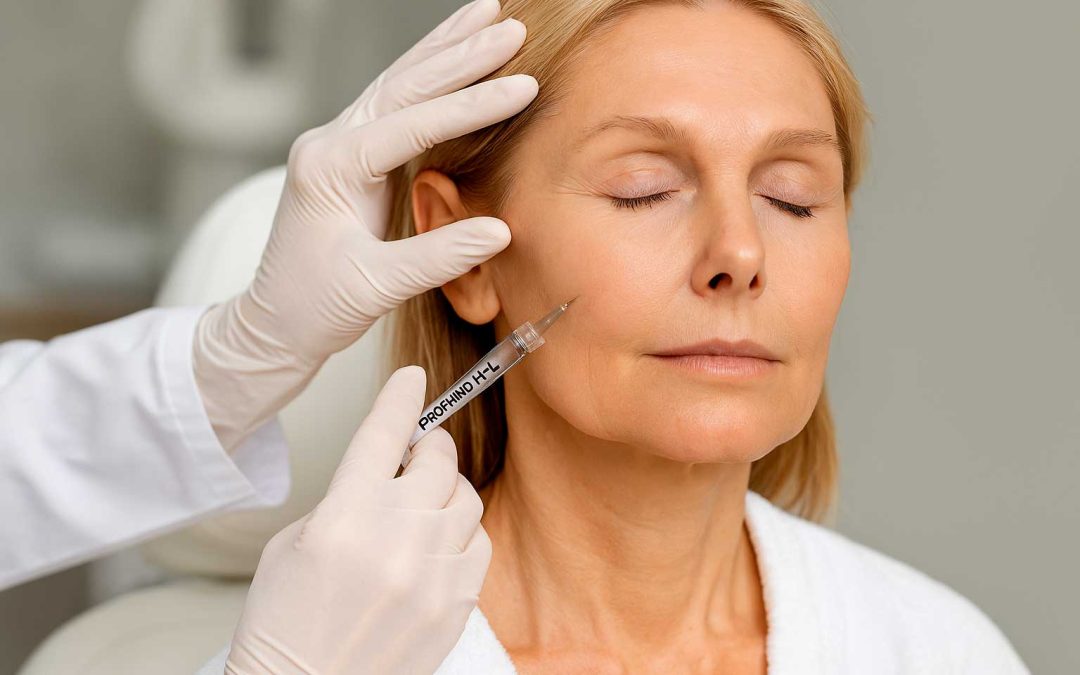
Profhilo H+L side effects are among the most searched concerns by aesthetic doctors and healthcare professionals when evaluating injectable bio-remodeling treatments. PROFHILO® H+L (32mg/2ml) is a CE-certified, non-crosslinked hyaluronic acid (HA) product designed to address skin laxity and improve hydration. While its safety profile is favorable, understanding its clinical behavior, potential side effects, and appropriate protocols is crucial for optimal results and patient trust.
This article provides an evidence-based, SEO-optimized guide for professionals who administer Profhilo, focusing on minimizing risks, managing adverse reactions, and reinforcing patient safety through structured treatment planning.
What Is Profhilo® H+L 32mg/2ml?
Profhilo® H+L is composed of:
- 32 mg High Molecular Weight HA (HMW-HA)
- 32 mg Low Molecular Weight HA (LMW-HA)
- 2 ml prefilled syringe
- No BDDE or chemical crosslinking
This hybrid formula (NAHYCO™ technology) creates stable hybrid cooperative complexes, which allow for slow release and deep tissue stimulation of collagen and elastin without creating volume.
Indications include:
- Facial skin laxity (cheeks, jawline)
- Neck and décolleté rejuvenation
- Hands and arms (off-label)
Manufacturer: IBSA Derma (Switzerland)
CE Certification: Class III (Europe)
Official site: https://www.ibsaderma.com
Common and Rare Side Effects of Profhilo H+L
Most side effects are mild and transient. Serious complications are rare but must be addressed with proper clinical action.
Table 1: Profhilo Side Effects and Frequencies
| Side Effect | Frequency | Onset/Duration | Notes |
|---|---|---|---|
| Swelling, redness | Very common | 12–48 hours | Typically self-resolving |
| Bruising | Common | Immediate or delayed | Cannula use may reduce incidence |
| Tenderness | Common | Up to 3 days | Localized to injection point |
| Nodules or lumps | Uncommon | 1–2 weeks | May resolve or need massage |
| Allergic reaction | Rare | Immediate | Requires antihistamines or corticosteroids |
| Infection or abscess | Very rare | Days post-injection | Needs antibiotic treatment |
| Vascular compromise | Extremely rare | Immediate | Emergency intervention required |
Source: IBSA Australia IFU PDF
Safety Mechanism and Why Profhilo Is Low Risk
- No cross-linking agents: Eliminates BDDE-related reactions
- Sterile single-use syringe reduces contamination
- Does not create volume, reducing migration and embolic risks
Study Reference:
Evangelista et al. (2020), “Use of a hybrid cooperative complex in skin remodeling” – Journal of Clinical and Aesthetic Dermatology
PubMed PMC7327616
Clinical Guidelines for Safe Administration
| Protocol Area | Clinical Recommendation |
| Injection Points | Bio Aesthetic Points (BAP) technique (5 points) |
| Needle Size | 29G x 13 mm (standard); cannula optional |
| Injection Depth | Subcutaneous |
| Volume per Point | 0.2 ml |
| Total Volume | 2 ml/session |
| Interval Between Sessions | 4 weeks (initial protocol) |
| Maintenance | Every 6–12 months |
Source: IBSA Derma Training Manual (2022)
How to Manage Profhilo Adverse Events
| Adverse Event | Management Strategy |
| Bruising/Swelling | Cold packs, arnica, avoid NSAIDs pre-treatment |
| Persistent nodule | Massage, hyaluronidase if unresolved |
| Allergic reaction | Antihistamines or corticosteroids, monitor vitals |
| Infection | Prescribe oral antibiotics, assess wound care |
| Vascular event | Hyaluronidase, warm compress, refer if needed |
Further reading:
Perfect Skin Studio – Profhilo Gone Wrong
Doctor Medica – Profhilo Side Effects
Combining Profhilo with Other Aesthetic Treatments
Profhilo can safely complement other modalities with proper timing.
Table 2: Recommended Intervals
| Treatment Type | Time Buffer Before/After Profhilo |
| Botulinum toxin | 1 week before or after |
| Laser treatments | ≥2 weeks after Profhilo |
| Radiofrequency/Microneedling | 1–2 weeks buffer |
| Chemical Peels | Avoid concurrent use |
| Fillers (crosslinked HA) | Minimum 2 weeks apart |
Source: AMWC 2022 Consensus Guidelines
Post-Treatment Patient Instructions
- Avoid makeup for 12 hours
- No saunas, sun exposure, or strenuous activity for 48 hours
- Expect small papules at injection points lasting up to 24 hours
- Do not massage the area unless directed
Providing written aftercare improves patient safety and satisfaction.
Summary Table: Profhilo H+L Clinical Overview
| Attribute | Detail |
| HA Content | 32 mg HMW + 32 mg LMW per 2 ml |
| Cross-linking agent | None (NAHYCO™ Technology) |
| Target indication | Skin laxity, hydration |
| Duration | 6 to 12 months |
| Common side effects | Swelling, redness, bruising |
| Rare complications | Nodules, allergic reactions, vascular issues |
| CE Certification | Yes (Class III, EU) |

Visual guide for aesthetic doctors: managing Profhilo H+L side effects and injection safety protocols.Final Notes for Professionals
Profhilo H+L is a cutting-edge injectable suitable for a wide range of patients when used by qualified professionals. It offers a strong safety profile thanks to its non-volumizing, non-crosslinked formulation. However, awareness of Profhilo H+L side effects, anatomy, and product behavior is essential to achieve high patient satisfaction and avoid clinical pitfalls.
Stay informed, document procedures, and maintain continuous professional development to ensure safety and effectiveness with bioremodeling injectables.
References
IBSA Derma Official Website: https://www.ibsaderma.com
IBSA Australia: Profhilo IFU PDF
PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7327616
Doctor Medica: https://www.doctormedica.co/blog/profhilo-side-effects
Perfect Skin Studio: https://perfectskinstudio.co.uk/blog/profhilo-gone-wrong
AMWC Best Practices (2022)
REVOLAX™ SUB-Q is a powerful and reliable filler when used correctly. Due to its structural nature, it is ideal for deep volumizing but requires:
- Deep anatomical understanding
- Careful patient selection
- Strict aseptic protocol
- Conservative dosing and aspirational technique
Its side effect profile is favorable in trained hands. Always educate patients clearly, and stay updated with complication management standards.
References and Resources
- Manufacturer: https://revolax.com
- Clinical Guide on Adverse Events: https://doi.org/10.2147/CCID.S80446
- Across Co. Ltd. Product Documentation
- ISAPS Safety Guidelines (2023)
- AMWC Best Injection Practices (2022)