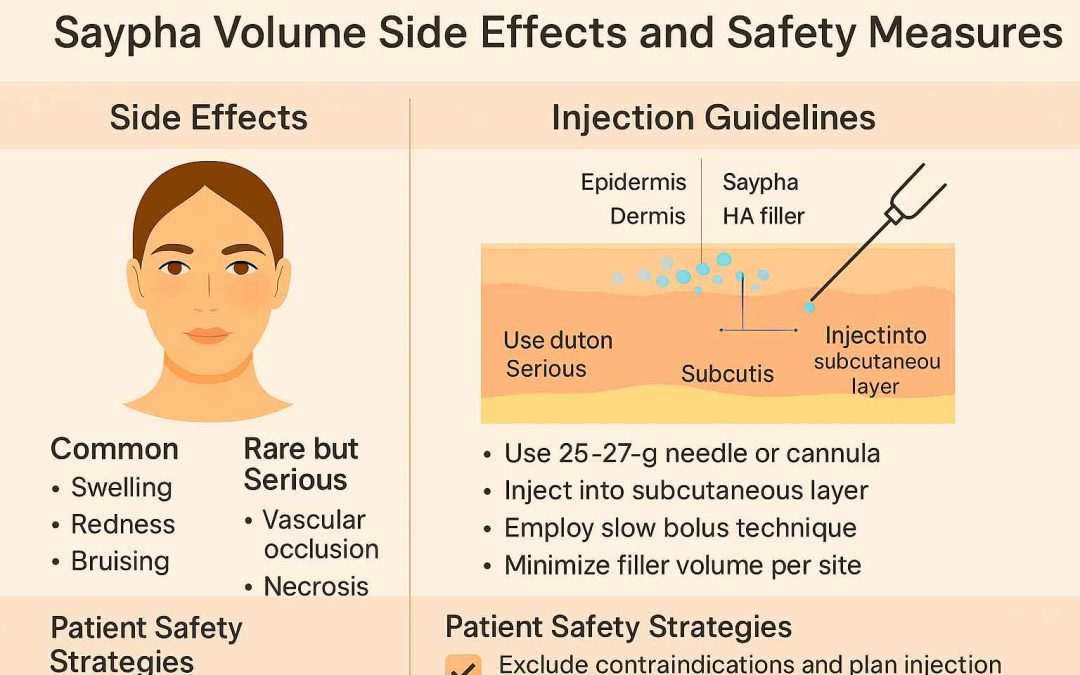
Saypha Volume side effects are among the top concerns for aesthetic doctors using dermal fillers in mid- and deep-volume restoration. Manufactured by Croma-Pharma GmbH, Saypha Volume (formerly known as Princess Volume) is a hyaluronic acid (HA) filler designed for restoring facial volume and treating deep wrinkles. This guide outlines key safety considerations, potential risks, injection techniques, and clinical data to assist medical professionals in delivering optimal patient outcomes.
What is Saypha Volume (23 mg/ml)?
Saypha Volume is a monophasic, cross-linked HA dermal filler indicated for:
- Deep facial wrinkles (e.g., nasolabial folds)
- Facial volume loss (cheeks, temples, chin)
- Mid-face augmentation
Product specifications:
- HA concentration: 23 mg/ml
- Cross-linking agent: BDDE (minimal residuals)
- Format: 1 x 1 ml prefilled syringe
- Lidocaine: Optional version available
Manufacturer: Croma-Pharma GmbH (Austria)
Certification: CE Class III medical device
Official site: https://croma.at/en/products/saypha-volume
Common and Rare Side Effects of Saypha Volume
Like all HA fillers, Saypha Volume is generally well tolerated but may present local or rare systemic adverse events.
Table 1: Saypha Volume Side Effects
| Side Effect | Frequency | Notes |
|---|---|---|
| Redness, swelling | Very common | Resolves within 2–3 days |
| Bruising | Common | Related to injection depth or needle size |
| Tenderness, itching | Common | Mild and self-limiting |
| Lumps or nodules | Occasional | May result from overcorrection or poor technique |
| Hypersensitivity reaction | Rare | Monitor for redness, warmth, systemic symptoms |
| Vascular occlusion | Very rare | Emergency; immediate protocol required |
Sources: Croma Safety Data, PubMed Review on HA Fillers
Injection Techniques and Anatomical Guidelines
Saypha Volume is designed for injection into the deep dermis, subcutaneous tissue, or supraperiosteal plane, depending on the area treated.
Table 2: Recommended Injection Techniques
| Treatment Area | Depth | Technique | Notes |
| Cheeks | Supraperiosteal | Bolus or fanning | Use cannula or needle 25–27G |
| Nasolabial folds | Mid to deep dermis | Linear threading or fanning | Avoid overfilling |
| Chin | Supraperiosteal | Bolus injections | Assess facial symmetry |
| Temples | Deep subcutaneous | Retrograde linear threading | Low volume, high precision |

Safety Protocols and Complication Management
Prevention strategies:
- Use aspiration before injection in high-risk zones
- Employ slow injection and minimal pressure
- Avoid vascular danger zones without anatomical training
Table 3: Adverse Event Management
| Complication | Prevention | Management |
| Vascular occlusion | Slow injection, aspiration | Hyaluronidase, warm compress, referral |
| Lumps/nodules | Proper depth, avoid overfilling | Massage, hyaluronidase if persistent |
| Inflammation/allergy | Screen patient history | Antihistamines, corticosteroids if needed |
Saypha Volume vs Other HA Fillers
| Attribute | Saypha Volume | Typical HA Filler |
| HA concentration | 23 mg/ml | 20–25 mg/ml |
| Cross-linking agent | BDDE (low residuals) | Varies |
| Particle structure | Monophasic, smooth | Varies |
| Longevity | 6–12 months | 6–12 months |
| Origin | Biofermentation | Common across HA fillers |
Patient Selection and Aftercare
Ideal candidates:
- Adults aged 30–65 with mid-face volume loss
- No active skin infections or autoimmune conditions
- Informed, realistic expectations
Aftercare instructions:
- Avoid intense heat (sauna, sun) for 48 hours
- No alcohol or exercise 24 hours post-treatment
- Gentle cleansing; no pressure on treated area
- Return if pain, discoloration, or swelling persists
Summary: Clinical Profile of Saypha Volume
| Attribute | Specification |
| Product name | Saypha Volume 23 mg/ml |
| Indications | Volume loss, facial contouring |
| Duration of effect | Up to 12 months |
| Reversible | Yes, with hyaluronidase |
| CE certification | Yes (Class III Medical Device) |
| Manufacturer | Croma-Pharma GmbH (Austria) |
| Suitable injection plane | Deep dermis, subcutaneous, supraperiosteal |
References
- Croma-Pharma Official Product Page
- PubMed: HA Filler Complication Review
- ISAPS Guidelines on Dermal Fillers